Nature Cardiovascular Research 2022:
A human cell atlas of the pressure-induced
hypertrophic heart
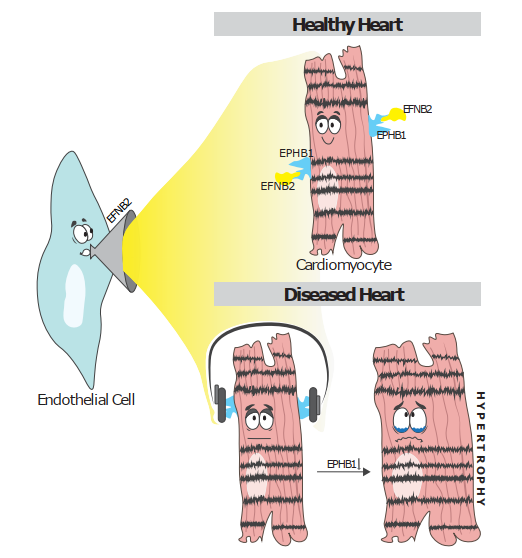
With a interdisciplinary team including clinical experts, bioinformaticians, and molecular biologists, we applied large-scale single-nuclei technologies to construct an atlas of the human hypertrophied heart. So far, research has mainly focused on the pathophysiology of cardiomyocytes, but the interplay of cardiomyocytes with other cells of the heart has recently gained attention. In our study, we found that intercellular crosstalk is crucial in driving disease progression with a striking reduction of communication between different cell types in a hypertrophied environment. Cardiomyocyte cross-talk was most strikingly diminished in hypertrophied hearts, especially regarding their communication with endothelial cells.
Several genes encoding Eph receptor tyrosine kinases, particularly EPHB1, were significantly downregulated in cardiomyocytes of the hypertrophied heart, which reduced EPHB1 activation via its ligand EFNB2, which is mainly expressed by endothelial cells. We therefore investigated the effects of EPHB1-EFNB2 signaling in-vitro and found that EFNB2 protected cardiomyocytes from hypertrophy, while silencing EFNB2 in endothelial cells was sufficient to induce hypertrophy and stress in co-cultured cardiomyocytes. In summary, our human cell atlas of the hypertrophied heart highlights the role of intercellular cross-talk in disease pathogenesis and provides a valuable resource for further studies.
Original publication:
Nicin, L., Schroeter, S.M., Glaser, S.F. et al. A human cell atlas of the pressure-induced hypertrophic heart. Nat Cardiovasc Res (2022). https://doi.org/10.1038/s44161-022-00019-7